BLOG: Advocating for a Cruelty-Free Future in Cosmetics Testing
By Audrey Pham, Legal Intern (2L - University of Pennsylvania Carey Law School)
This past March, Washington became the 12th state in the U.S. to ban the sale of cosmetics tested on animals. The bill, H.B. 1097, was first introduced in 2022 before being signed into law by Washington Governor Jay Inslee. The legislation makes it unlawful for a manufacturer to “sell or offer for sale” cosmetics in Washington “if the cosmetic was developed or manufactured using cosmetic animal testing that was conducted or contracted for by the manufacturer or any supplier of the manufacturer.” The legislation is effective January 1, 2025.
Washington’s ban on cosmetics animal testing follows similar laws passed by California, Hawai’i, Illinois, Louisiana, Maine, Maryland, Nevada, New Jersey, New York, Oregon, and Virginia state legislatures. Globally, 45 countries have passed laws limiting or prohibiting the use of animals in cosmetics testing, including the entire European Union.
While these individual state actions against cosmetics animal testing are promising, the country-wide bans around the world make one thing clear: the United States as a country needs to do better. Presently, no federal law exists to protect animals subjected to cosmetics testing. The Humane Cosmetics Act of 2023 (H.R. 5399) was first introduced in the U.S. House of Representatives back in 2015. The Act is a bipartisan bill that seeks to end the use of animals in cosmetics testing and the sale or transport of animal-tested cosmetics across the U.S. Instead, only cosmetics approved under scientifically advanced safety assessments and technology would enter the American market.
Eight years later, in 2023, the Act has been reintroduced in the U.S. House of Representatives and still has yet to be enacted into legislation. Yet, the Act’s influence on state legislatures is profound. Many of the states’ bans on animal-tested cosmetics are modeled off the Act, Washington included. The reintroduction of this Act reflects the longstanding concern with cosmetics animal testing practices. As Representative Don Beyer, one of the politicians responsible for the bill’s introduction and reintroduction, puts it, “Cosmetics testing on animals is cruel, unnecessary, and outdated … Much of the cosmetics industry has already moved to more scientifically sound methods that do not result in animal cruelty. The Humane Cosmetics Act would outlaw an obsolete and inhumane practice without damaging American businesses.”
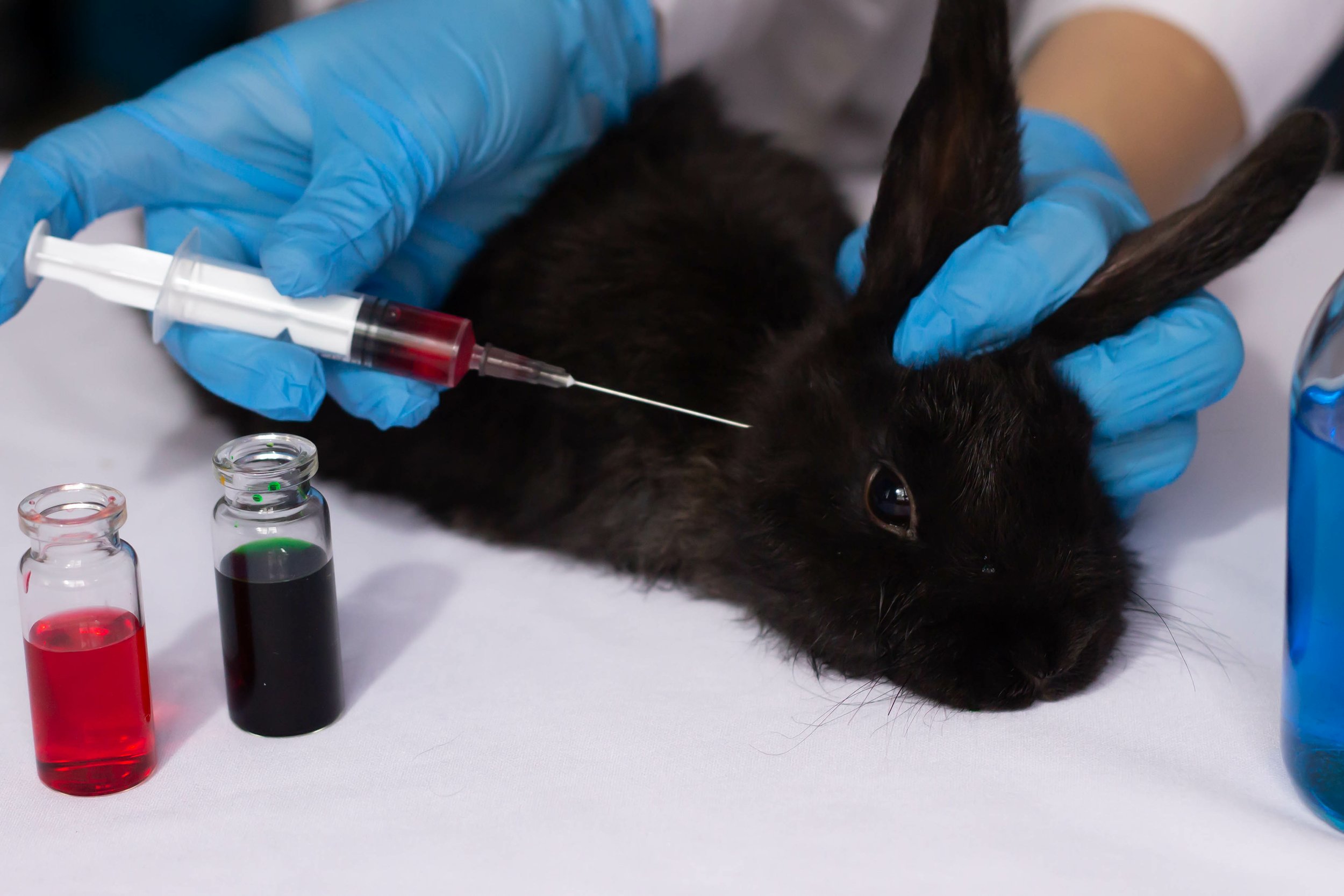
The pain and suffering that cosmetics testing inflicts on animals is immense. When we think of cosmetics, we most commonly think of makeup products. The unfortunate reality is that cosmetics includes a wide range of products beyond makeup. The Food and Drug Administration (FDA) defines cosmetics as “articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body...for cleansing, beautifying, promoting attractiveness, or altering the appearance.” This includes skin moisturizers, perfumes, nail polishes, shampoos, and deodorants, to name a few. This broad definition results in large amounts of cosmetics testing on animals, and of many kinds.
One of the most common cosmetics animals testing procedures is dermal penetration tests, also referred to as skin absorption tests. The stated purpose of dermal penetration tests is twofold: 1) to determine the rate at which a given chemical will penetrate the skin, and 2) to assess what happens to the chemical when it is inside the body. Rats are the most common animal used for such tests. While the rats are still alive, researchers shave their backs and spread the chemical across their bare skin. The rats are then killed at different time intervals following the chemical exposure for the purposes of analyzing their blood, tissue, and excrement. Pain relief is not provided to the rats.
Despite the prevalence of dermal penetration tests, the benefits of the research findings are questionable. There are significant differences in the skin absorption rates of rat versus human skin. A study comparing the dermal absorption rates of in vitro (research done in a laboratory dish or test tube) rat and human skin versus in vivo (research done directly on a living organism) rat skin observed that rat skin was more permeable to all tested substances than human skin – finding that in vivo rat studies yield significantly overestimated and inaccurate risk assessments for humans. From that, the study concluded that rat skin is more permeable than human skin.
Skin sensitization testing is another common cosmetics animal testing method. Like dermal penetration tests, this method also involves live animals. The test is designed to evaluate the ability of antigens to cause hypersensitivity, such that researchers can determine whether repeated exposure of a substance to the skin will cause allergic reactions. The “gold standard” for skin sensitization testing is the Magnusson & Kligman Guinea Pig Maximization Test (GPMT), wherein guinea pigs are injected with product formulations and materials (some of which are extracts of solid materials) multiple times around their head and tail. The guinea pigs are monitored for their allergic reactions, including redness and swelling, as well as burns or infections on the skin creating skin wounds, dead tissue, and dried secretions. If there is irritation in the control group, all test and control guinea pigs are again subjected to the skin sensitization test on the untreated side of their bodies and its painful skin reactions, a process called “rechallenge.” However, because GPMT was developed to identify weaker allergens by maximizing exposure and inducing hyperirritability, the test is prone to producing inaccurate results – namely false-positive irritant reactions that overstate allergenicity to test substances. This predictive test method yields numerous probable inaccuracies and, even if the requisite number of guinea pigs tested positive for product sensitization, one question remains: “has the substance been correctly identified in terms of human standard?”
Among the most widely condemned cosmetics animal tests is acute toxicity (LD50) testing, also known as lethal dose testing. The purpose of the test is to estimate the test substance dosage that will produce a 50% death in a given animal species, which is, in turn, used to estimate the potential toxicity that a chemical may pose for humans. The test is typically performed on mice, rats, pigeons, quail, and fish. Acute toxicity testing has a long history with many different methods being introduced throughout the 1900s – some involving upwards of 100 animals per experiment. Researchers observe the animals for signs of tremors, arching and rolling, muscle spasms, and writing, to name a few. In every iteration of these acute toxicity testing methods, the animals’ deaths are a deliberate result of the experiment.
Yet, interestingly enough, acute toxicity testing presents significant limitations due to the quantitative and qualitative difference between animals and humans. Many studies reflect how animal findings often produce inaccurate predictions of human toxicity. For example, one review of 76 animal studies found that only about 20% animal findings were contradicted in humans and only 37% were replicated in humans. The results also vary widely from species to species depending on the animal’s age, sex, and diet. Furthermore, the results are questionable because the test itself is scientifically inconsistent in methodology. Experiment procedures such as the extent of food deprivation prior to chemical dosing, temperature, and caging vary from laboratory to laboratory. Oftentimes, such toxicity tests fail to predict severe and fatal toxicity responses in humans. This is illustrated by the failure rates in drug development – approximately 89% of novel drugs fail in clinical trials, half of which are due to unanticipated human toxicity despite safety approvals from animal toxicity testing.
Consumer attitudes towards cosmetics animal testing are overwhelmingly united, according to a nationwide survey of 1,000 Americans ranging in age and political party. This survey reveals an overall cross-generational opposition to cosmetics animal testing – 65% of Americans aged 18-34, 72% of Americans aged 35-49, 76% of Americans aged 50-64, and 75% of Americans aged 65 and older being opposed. There is also unified support for a federal law banning cosmetics animal testing regardless of political party affiliation. Findings from the survey indicate that 83% of Democrats, 72% of Republicans, and 80% of Independents “support” or “strongly support” a federal end to the use of animals in cosmetics testing.
Both across the United States and abroad, people are taking note of both the animal suffering that cosmetics testing causes and the wealth of safe non-animal cosmetics alternatives – making it more obvious than ever that it is time for a federal-level switch to non-animal cosmetics testing. As the largest market for cosmetics in the world, the United States must eliminate the unethical and outdated cosmetics animal testing practices guarantee the safety of these animals nationwide by signing the Humane Cosmetics Act into law. If passed, the Act will prohibit 1) the evaluation of cosmetics products via animal testing, 2) the sale and/or transport of cosmetics products using animal testing, and 3) the use of animal-testing evidence in establishing the safety of a cosmetic product or ingredient regulated by the FDA.
The tides appear to be changing. Numerous non-animal cosmetics testing alternatives, as well as cruelty-free cosmetic product alternatives, have emerged due to scientific and technological advancements. Examples include in vitro permeation experiments, which use models of human skin tissues and/or samples of cells, rather than entire living model organisms that are killed soon after.
Contact your representatives and senators today requesting their support of the Humane Cosmetics Act